Selected publications:
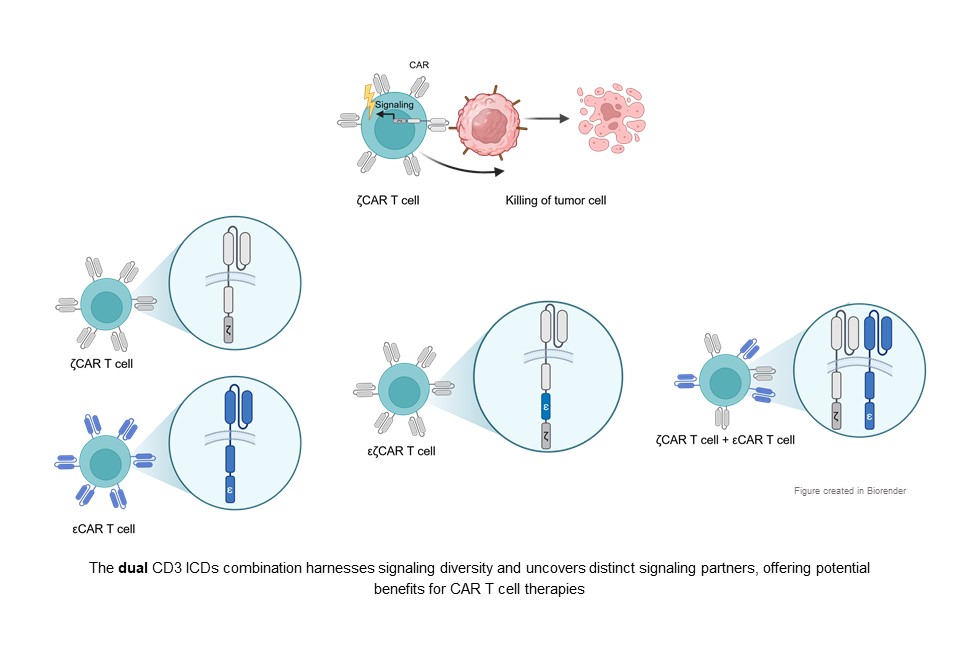
Velasco Cárdenas, R. M., Brandl, S. M., Meléndez, A. V., Schlaak, A. E., Buschky, A., Peters, T., Beier, F., Serrels, B., Taromi, S., Raute, K., Hauri, S., Gstaiger, M., Lassmann, S., Huppa, J. B., Boerries, M., Andrieux, G., Bengsch, B., Schamel, W. W., & Minguet, S. (2023). Harnessing CD3 diversity to optimize CAR T cells. Nature immunology: 24(12), 2135–2149. doi.org/10.1038/s41590-023-01658-z
Tomisch, J., Busse, V., Rosato, F., Makshakova, O. N., Salavei, P., Kittel, A. S., Gillon, E., Lataster, L., Imberty, A., Meléndez, A. V., & Römer, W. (2023). A Shiga Toxin B-Subunit-Based Lectibody Boosts T Cell Cytotoxicity towards Gb3-Positive Cancer Cells. Cells: 12(14), 1896. doi.org/10.3390/cells12141896
Xu, M., Antonova, M., Salavei, P., Illek, K., Meléndez, A. V., Omidvar, R., Thuenauer, R., Makshakova, O., & Römer, W. (2023). Dimeric Lectin Chimeras as Novel Candidates for Gb3-Mediated Transcytotic Drug Delivery through Cellular Barriers.Pharmaceutics: 15(1), 225. doi.org/10.3390/pharmaceutics15010225
Rosato, F., Pasupuleti, R., Tomisch, J., Meléndez, A. V., Kolanovic, D., Makshakova, O. N., Wiltschi, B., & Römer, W. (2022). A bispecific, crosslinking lectibody activates cytotoxic T cells and induces cancer cell death. Journal of translational medicine: 20(1), 578. https://doi.org/10.1186/s12967-022-03794-w
Meléndez, A. V., Velasco Cárdenas, R. M., Lagies, S., Strietz, J., Siukstaite, L., Thomas, O. S., Tomisch, J., Weber, W., Kammerer, B., Römer, W., & Minguet, S. (2022). Novel lectin-based chimeric antigen receptors target Gb3-positive tumour cells. Cellular and molecular life sciences: CMLS, 79(10), 513. doi.org/10.1007/s00018-022-04524-7
Frensch, M., Jäger, C., Müller, P. F., Tadić, A., Wilhelm, I., Wehrum, S., Diedrich, B., Fischer, B., Meléndez, A. V., Dengjel, J., Eibel, H., & Römer, W. (2021). Bacterial lectin BambL acts as a B cell superantigen. Cellular and molecular life sciences: CMLS, 78(24), 8165–8186. doi.org/10.1007/s00018-021-04009-z
Brandel, A., Aigal, S., Lagies, S., Schlimpert, M., Meléndez, A. V., Xu, M., Lehmann, A., Hummel, D., Fisch, D., Madl, J., Eierhoff, T., Kammerer, B., & Römer, W. (2021). The Gb3-enriched CD59/flotillin plasma membrane domain regulates host cell invasion by Pseudomonas aeruginosa. Cellular and molecular life sciences: CMLS, 78(7), 3637–3656. doi.org/10.1007/s00018-021-03766-1
Østevold, K., Meléndez, A. V., Lehmann, F., Schmidt, G., Aktories, K., & Schwan, C. (2017). Septin remodeling is essential for the formation of cell membrane protrusions (microtentacles) in detached tumor cells.Oncotarget: https://doi.org/10.18632/oncotarget.20805