Ultraviolet (UV) radiation from sunlight causes sunburn and increases the risk of skin cancer by damaging our DNA as well as our RNA. Researchers at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg have now unveiled a cellular shield that protects cells from the harmful effects that UV-induced RNA damage has.


Protective mechanism against UV-induced cell damage discovered
Freiburg researchers find that DHX9 stress granules protect cells from UV-induced RNA damage
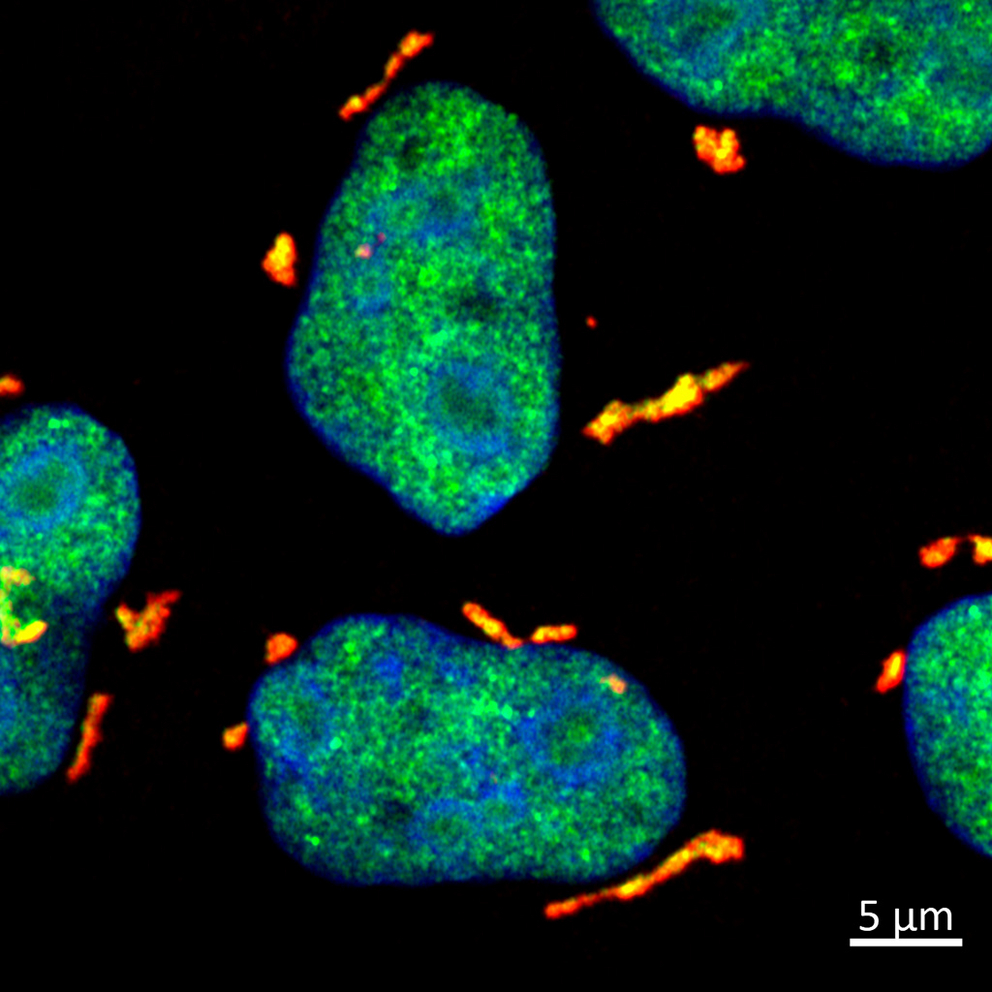
Fluorescence microscopy image of cells with UV-induced RNA damage. The proteins DHX9 and G3BP1 are labelled in green and red, respectively, and co-localize in stress granules (yellow). Image: Asifa Akhtar/MPI-IE
During the process of cell division, daughter cells inherit genetic material and other molecules. This inheritance includes both, beneficial components and potentially harmful mutations and damaged molecules. How daughter cells manage and mitigate the effects of this harmful inheritance has been unknown. A study led by Prof. Dr. Asifa Akhtar, Director at the Max Planck Institute of Immunobiology and Epigenetics and member of the Cluster of Excellence CIBSS at the University of Freiburg, has now revealed a sophisticated mechanism by which daughter cells protect themselves from UV-damaged RNA inherited from mother cells.
Ultraviolet (UV) radiation is the most energetic component of sunlight. Although UV is known to damage DNA and contribute to skin cancer, its effect on another vital molecule, RNA, often goes unnoticed.
While testing the cellular response to various stressors, the researchers noticed something intriguing: after UV exposure, a protein called DHX9 gathered into droplet structures within the cell's cytoplasm. “DHX9 is an enzyme that normally resides in the nucleus and has the ability to bind RNA. Finding this protein forming droplets outside the nucleus left us really astonished. It's like finding a giant snowball in the desert,” says Akhtar.
DHX9 Granules trap damaged RNA
The researchers initially thought that the DHX9 granules protected against DNA damage. “Contrary to this hypothesis, we found that DHX9 granules were not triggered by various forms of DNA damage stimuli. And this prompted us to dig into the real trigger,” says Yilong Zhou, the study's first author. Therefore, the team developed a droplet extraction method to isolate these granules directly from cells and analyse their content.
Surprisingly, the team found that the DHX9 granules were packed with damaged RNA. “The damaging effect of UV light on RNA is frequently underestimated, overshadowed by its impact on DNA. Now, we discovered an elegant mechanism by which cells can segregate and neutralize harmful UV-damaged RNA with the help of DHX9 granules,” explains Akhtar. Cells rapidly trap the damaged RNA molecules in DHX9 granules, preventing them from causing further damage.
Granules are formed after cell division
“What fascinated us even more was the observation that cells with DHX9 granules always appeared in pairs, indicating that they are not formed in the original UV-damaged mother cell but later on in the newly born daughter cells,” says Zhou. The hypothesis is confirmed by live cell video imaging. “You can literally see that DHX9 normally resides in the nucleus, but shortly after cell division, when the two daughter cells have formed, it gathers into droplets in the cytoplasm,” Zhou continues. Interestingly, preventing the formation of DHX9 granules in daughter cells lead to cell death, highlighting the ability of daughter cells to spot and stash away their progenitors’ damaged RNA into DHX9 granules. “This process is like wiping the slate clean, preparing them to begin their own journey as a cell without dragging along the baggage from the previous generation,” says Akhtar.
Conditions such as sunburn, neurodegenerative disorders and cancer are closely linked to disruptions in RNA balance and irregularities in the cell cycle. The new findings therefore open up new possibilities for medical research: “A better understanding of how a newly generated cell selectively recognizes and degrades damaged RNA could lead to new therapeutic targets for diseases characterised by RNA mismanagement or dysregulation of the stress response,” explains Akhtar.
Original publication
Zhou Y, Panhale A, Shvedunova M, Balan M, Gomez-Auli A, Holz H, Seyfferth J, Helmstädter M, Kayser S, Zhao Y, Erdogdu NU, Grzadzielewska I, Mittler G, Manke T and Akhtar A (2024). RNA damage compartmentalization by DHX9 stress granules. In: Cell. DOI: 10.1016/j.cell.2024.02.028
CIBSS-Profile of Prof. Dr. Asifa Akhtar
Original press release on the MPI-IE website



