The surface of B cells is covered with antigen receptors with which they recognize invading pathogens such as bacteria and viruses. When a B cell receptor binds to an antigen, that is, to a foreign structure, the B cell is activated and triggers the production of antibodies. Antibodies are essential for our survival and protect us against severe diseases from infections with pathogens such as COVID-19. Vaccinations have a protective effect as they activate antigen receptors, thereby triggering an immune response. An international team of researchers from the Cluster of Excellence CIBSS of the University of Freiburg and Harvard Medical School, USA, has now published the exact molecular structure of an IgM-type B cell receptor. Their findings indicate that the receptor on the surface of the B cell interacts with further receptors, thus controlling its signal transduction. The study was published in the renowned journal Nature.
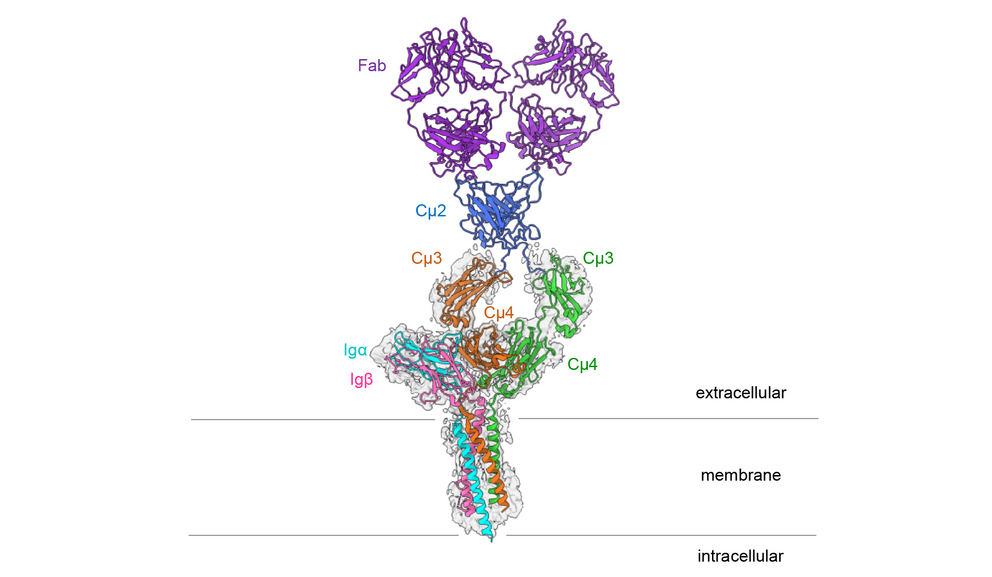
Connection of Signaling Subunits with the Immunoglobulin
The B cell antigen receptor consists of an antibody bound to the cell membrane and two smaller proteins, Ig alpha and Ig beta. These smaller subunits pass on signals to the inside of the cell as soon as the B cell receptor identifies a pathogen. “Exactly how these signaling subunits are connected with the immunoglobulin was previously unknown,” says Prof. Dr. Michael Reth from the University of Freiburg’s Faculty of Biology, who has been conducting research on the receptor for over 30 years and originally discovered its signaling subunits. He is a member of the Cluster of Excellence CIBSS – Centre for Integrative Biological Signalling Studies and co-director of the Cluster of Excellence BIOSS. “For a long time, we did not have the technical possibilities to study the exact structure of membrane proteins. Now, cryo-electron microscopy has enabled us to create a high-resolution image of the B cell receptor,” says Reth.